lipax™: NEOliposomal intracavitary paclitaxel technology for targeted chemotherapy
LiPax is a proprietary neoliposomal intracavitary paclitaxel drug delivery platform designed to enhance the therapeutic index of proven oncology agents, which results in more effective treatments for intracavitary cancers. LIPAC’s lead drug candidate, LiPax™, is a locally delivered formulation of the well-established chemotherapy drug, paclitaxel. It has completed a Phase 1-2a clinical trial for non-muscle invasive bladder cancer (NMIBC) and has demonstrated impressive clinical activity while being very well tolerated and safe. LiPax is also in preclinical development for malignant pleural effusion (MPE), intraperitoneal carcinoma (ovarian) and upper tract urothelial carcinoma (UTUC).
Paclitaxel, an agent known to be highly active in systemic application for urothelial carcinomas, is lipophilic and not solubilized in the acidic environment of the bladder. It is known to cause systemic toxicity resulting in severe side effects such as peripheral neuropathy, hearing loss, low blood count, and hair loss among others. Paclitaxel has not been formulated or approved to be used intravesically in its existing formulations. By utilizing LiPax to enhance permeation and solubility, we have overcome the barriers to intravesical delivery of paclitaxel. LIPAC has conducted preclinical and clinical studies which demonstrated superior efficacy of intracavitary delivery, and we attribute the success of this drug delivery model to the following key differentiators:
LETHALITY
The effectiveness of paclitaxel against various types of cancer is well established. Laboratory tests have demonstrated that formulating paclitaxel as LiPax enhances its lethality by a factor of more than 200-fold against T24 human cancer cells compared to Mitomycin C (MMC). Consequently, smaller concentrations of LiPax will be required to achieve the same or greater response rates in patients.
PERSISTENCE
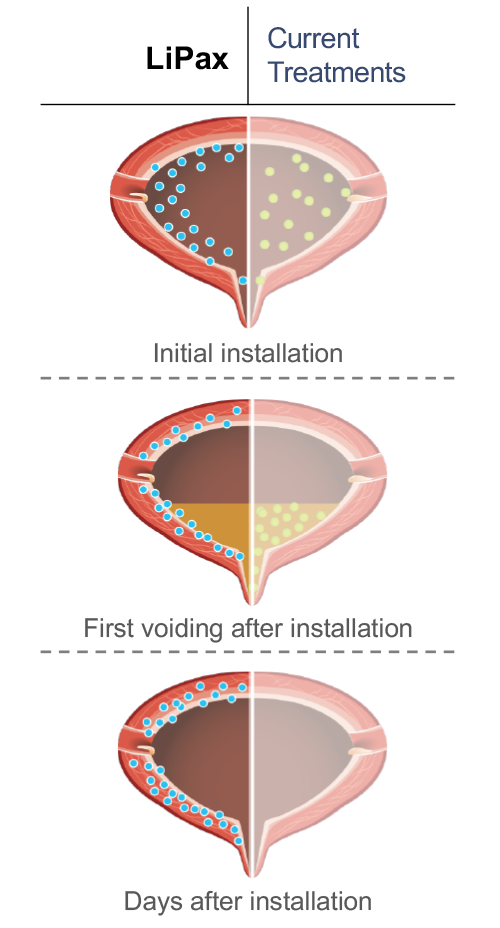
Current intravesical treatments, such as MMC, have not been formulated or approved for the hostile and acidic bladder environment and as such have very limited effectiveness after a patient’s voiding of the bladder urine. Unlike existing treatments, LiPax is lipophilic and adheres to the urothelium which enables adherence of LiPax on the bladder wall, persisting as a reservoir of high concentration drug. Studies have shown that liposomes can persist on the bladder wall for multiple days after instillation and provide patients with sustained exposure to the active chemotherapy agent long after treatment.
PENETRATION
In contrast to current treatments that are mostly hydrophilic, LiPax’s lipophilic structure penetrates deep into the target tissue (e.g. bladder wall). Ex vivo studies have confirmed lethal concentrations of paclitaxel extending into the muscle layer but not beyond the bladder wall. Promising complete response rates have been achieved in nude mice studies, confirming effective localized targeting of tumor cells without any systemic exposure or toxicity.
technology overview
Formulation components self-assemble into liposomes upon addition of sterile water diluent
Self-assembly is driven by physiochemical properties of surfactant to maximize volume and minimize surface area
The active product ingredient, paclitaxel, intercalates in the lipid rich region of the phospholipid
Random motion and composition of the liposomes assist with adhesion to the urothelial wall
Paclitaxel is off-loaded into the target tissue and penetrates deep into the bladder wall
Formulation benefits
Paclitaxel is highly active against metastatic bladder cancer with in vitro studies showing it is 200-fold more effective than MMC
Enhanced tolerability and no measurable systemic toxicity
Liposomal formulation adheres to the bladder wall targeting bladder cancer cells and penetrating the urothelium
Optimized off-load-kinetics and intratumoral concentration relative to Abraxane
Closed packaging system allows for less complicated storage and ease of handling, and is ready for use after adding the sterile water diluent
In vitro studies indicated LiPax penetrates the bladder wall more effectively than other paclitaxel formulations
LiPax is presented in a container as a dry powder and the simple manufacturing process mitigates the potential risks of large scale production
LiPax can be stored in office by physicians and safely transported without the degradation issues common in liposomal formulations
At time of treatment, water for injection (WFI) is added into the closed system to hydrate LiPax, forming a liposomal dispersion for intravesical instillation
Higher drug load in liposomal formulation results in increased potency against cancer cells
Paclitaxel is a known chemical entity with a well-established efficacy and safety profile. Given this and previous regulatory approvals, LIPAC has a streamlined 505(b)2 pathway agreed to with the U.S. Food and Drug Administration